What is it
The pH scale is a means of defining the strength of an acid or alkali. Whether a solution is acidic or basic (alkaline) depends on the concentrations of dissociated hydrogen ions (H+) or hydroxyl ions (OH-). If H+ are dominant the solution will be acidic, if OH- are dominant the solution will be alkaline. The more H+ or OH- the stronger the reaction. Strong acidity or alkalinity can destroy biological tissue, dissolve rocks and corrode metals. The more H+ or OH–, the stronger the reaction. Pure water is neutral, with a pH of 7, and has equal amounts of H+ and OH–. Coca-Cola, wine and citrus juices typically have pH 3–4, strong acids have pH <2.
The pH scale runs from 0 to 14 being defined as pH = –log10[H+]. In a solution at 25°C the product of the H+ and OH– is constant, so that [H+] × [OH–] = 10–14. Where H+ and OH– are present in equal concentration the solution is neutral and has a pH of 7.0. Acid solutions have an excess of H+ and a pH <7, basic (alkaline) solutions have an excess of OH– ions and a pH >7. Soil acidity differs from soil pH as the H+ ions in solution are only a small proportion of the total, the others being present on exchange sites. The number of exchange sites is related to the cation exchange capacity and buffering capacity of a soil. To alter soil acidity it is necessary to add sufficient neutralising material (usually lime, CaCO3), to counter the soil acidity. As the H+ are replaced by Ca2+, so the pH rises, also reflected in the rise in Base Saturation (cf.).
Soil pH also influences the solubility and availability of a wide range of compounds in soil. Some metals, Aluminium for instance, are more soluble at low pH and can become toxic to plants. In the analytical laboratory, pH is most reliably determined in a soil slurry of 1 part soil to 2.5 parts water, with H+ ion concentration measured using a glass electrode. Portable field pH meters are available, as well as test kits that use indicator solutions to estimate pH by colour matching.
How to interpret it
Pasture crops
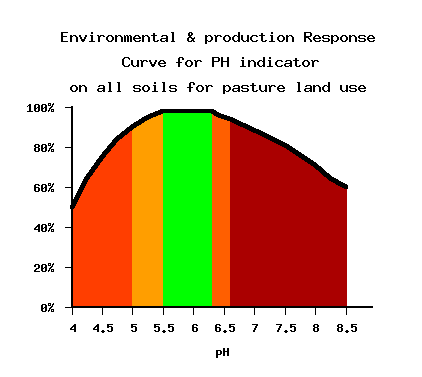
Grass-clover pastures perform best at slightly below neutral pH and the target values reflect these agronomic requirements.
 Alkaline pH 7–8. Well above optimal pH for pasture growth, loss of desirable
pasture species.
Alkaline pH 7–8. Well above optimal pH for pasture growth, loss of desirable
pasture species.
 Near neutral, pH 6.5–7.0 Higher than necessary for productive pasture and above
optimal for growth.
Near neutral, pH 6.5–7.0 Higher than necessary for productive pasture and above
optimal for growth.
 Optimal, pH 5.5–6.5. Generally recommended pH for productive
rye-grass white clover pastures
Optimal, pH 5.5–6.5. Generally recommended pH for productive
rye-grass white clover pastures
 Slightly acid, pH 5.0–5.5. Supports more productive grass species
and some clover but yields will be below optimal.
Slightly acid, pH 5.0–5.5. Supports more productive grass species
and some clover but yields will be below optimal.
 Very acid: Soils below pH 5 are strongly acid and usually support only the poorest
grasses with low nutrient contents. Clover will not establish.
Very acid: Soils below pH 5 are strongly acid and usually support only the poorest
grasses with low nutrient contents. Clover will not establish.
Native/Pine
Most indigenous forest species and Pinus radiata used for plantation forestry will grow well at much lower pH than pasture species. The optimal band for forestry is therefore wider and more acidic than for pastures.
 Alkaline, pH 6.5–7. Most forest species perform best under more
acidic conditions, and alkaline to neutral soils are not necessary for productive
forest.
Alkaline, pH 6.5–7. Most forest species perform best under more
acidic conditions, and alkaline to neutral soils are not necessary for productive
forest.
 Near neutral, pH 6–6.5. Above the pH needed for optimal growth
of native and plantation species. Such pH may be found when pastures
are converted to forestry, and reflect liming in earlier years for pasture production.
Near neutral, pH 6–6.5. Above the pH needed for optimal growth
of native and plantation species. Such pH may be found when pastures
are converted to forestry, and reflect liming in earlier years for pasture production.
 Optimal, pH 4.5–6. The optimal range for P. radiata plantations.
Many plantations have been planted on such soils without any pH modification.
Optimal, pH 4.5–6. The optimal range for P. radiata plantations.
Many plantations have been planted on such soils without any pH modification.
 Slightly acid, pH 4-4.5. Native species such as beech often grow in such acid soils,
but it is below the pH range preferred by P.radiata.
Slightly acid, pH 4-4.5. Native species such as beech often grow in such acid soils,
but it is below the pH range preferred by P.radiata.
 Very acid: Soils below pH 4 are strongly acid and even tolerant tree species will
show reduction in growth.
Very acid: Soils below pH 4 are strongly acid and even tolerant tree species will
show reduction in growth.
Cropping/Horticulture
Most arable and horticultural crops have a high lime requirement and perform best at slightly acid to neutral pH. Some crops have highly specific needs (e.g. blueberry requires acid conditions, truffles need highly alkaline soil), and we provide only general guidelines. Soils should also be sampled to greater depth than is generally used for pastures. Readers are referred to crop specific guides for more information (e.g. Clark et al. 1986).
 Alkaline, pH 7.1–8. Suitable for tolerant species such as lettuce
and celery.
Alkaline, pH 7.1–8. Suitable for tolerant species such as lettuce
and celery.
 Near neutral pH 6.3–7.1. Suitable for a more limited range of crops e.g. leeks,
celery, brocolli, some stonefruit.
Near neutral pH 6.3–7.1. Suitable for a more limited range of crops e.g. leeks,
celery, brocolli, some stonefruit.
 Optimal pH 5.8–6.3. Suitable for a wide range of crops e.g. citrus,
avocado, potato, tomato, carrots, cereals, squash, pipfruit.
Optimal pH 5.8–6.3. Suitable for a wide range of crops e.g. citrus,
avocado, potato, tomato, carrots, cereals, squash, pipfruit.
 Slightly acid pH 5.2–5.8. Suitable for some crops such as potato, water melon,
strawberry.
Slightly acid pH 5.2–5.8. Suitable for some crops such as potato, water melon,
strawberry.
 Very acid pH 4.5–5.2. Too acidic for many crops but acceptable for acid tolerant
species such as blueberry.
Very acid pH 4.5–5.2. Too acidic for many crops but acceptable for acid tolerant
species such as blueberry.
How to improve it.
Under New Zealand conditions most soils will acidify slowly with time, as ions are removed and leached preferentially from soil. Acidity is modified by applying crushed lime stone (calcium carbonate, CaCO3). Rarely, the more soluble quicklime (CaO or slaked lime Ca(OH)2 may be applied for a more rapid reaction. Different soils require different amounts of lime to obtain the same shift in pH. This is termed the lime requirement of a soil, and depends on the buffering capacity. For most ash, pumice and sedimentary soils 1 tonne of ground limestone per ha will raise the pH by about 0.1 unit. Most pastures are topdressed but the reaction will be more rapid if the lime can be incorporated into the soil by cultivation. Following heavy applications, the pH may continue to change for as long as a year as the lime slowly dissolves. Most nutrient budgeting applications will advise on lime requirements based on soil type and field pH levels.
Technical details
Soil pH is normally measured in the laboratory using a soil slurry in water. The concentration of H+ is measured using a calibrated glass electrode and a electronic meter.
Colorimetric tests are available for on-farm and home garden use, these rely on a colour change when an indicator dye solution is mixed with the correct proportions of soil. The pH is estimated from a colour chart.
Reference material
Clarke, C. J., Smith, G. S., Prasad, M, and Cornforth, I. S 1986. Fertiliser recommendations for Horticultural Crops.. First Edition pp.1-70. Wellington, Ministry of Agriculture and Fisheries.
During, C. (1984) Fertilisers and Soils in New Zealand Farming. Third Revised Edition. P.D. Hasselberg, Government Printer, Wellington.
McLaren, R.G. and Cameron, K.C. 1996 Soil Science. Revised Ed., 1-304, Auckland, Oxford University Press.
Roberts, A. H. C and Morton, J. D (Eds.) 1999. Fertiliser Use on New Zealand Dairy Farms. Revised, pp.1-37. Auckland, New Zealand. New Zealand Fertiliser Manufacturers Association.